AMI463
Investigational, brain-penetrant, first-in-class, highly selective small molecule inhibitor of the Cell adhesion molecule (CAM)-related downregulated by oncogenes (CDON)
AMI463 has received Orphan Drug Designation (ODD) from the European Medicines Agency (EMA) and from the US Food and Drug Administration (FDA) for the treatment of soft tissue sarcomas (STS). Additionally, AMI463 has obtained the Rare Pediatric Disease Designation (RPDD) from the FDA for the treatment of pediatric rhabdomyosarcoma, the most common STS in the pediatric population.
Soft tissue sarcomas represent a rare and heterogeneous group of tumors, arising in embryologically derived mesenchymal connective tissues. Advanced STS is associated with a poor prognosis, and treatment options are limited to chemotherapy.
First-in-class
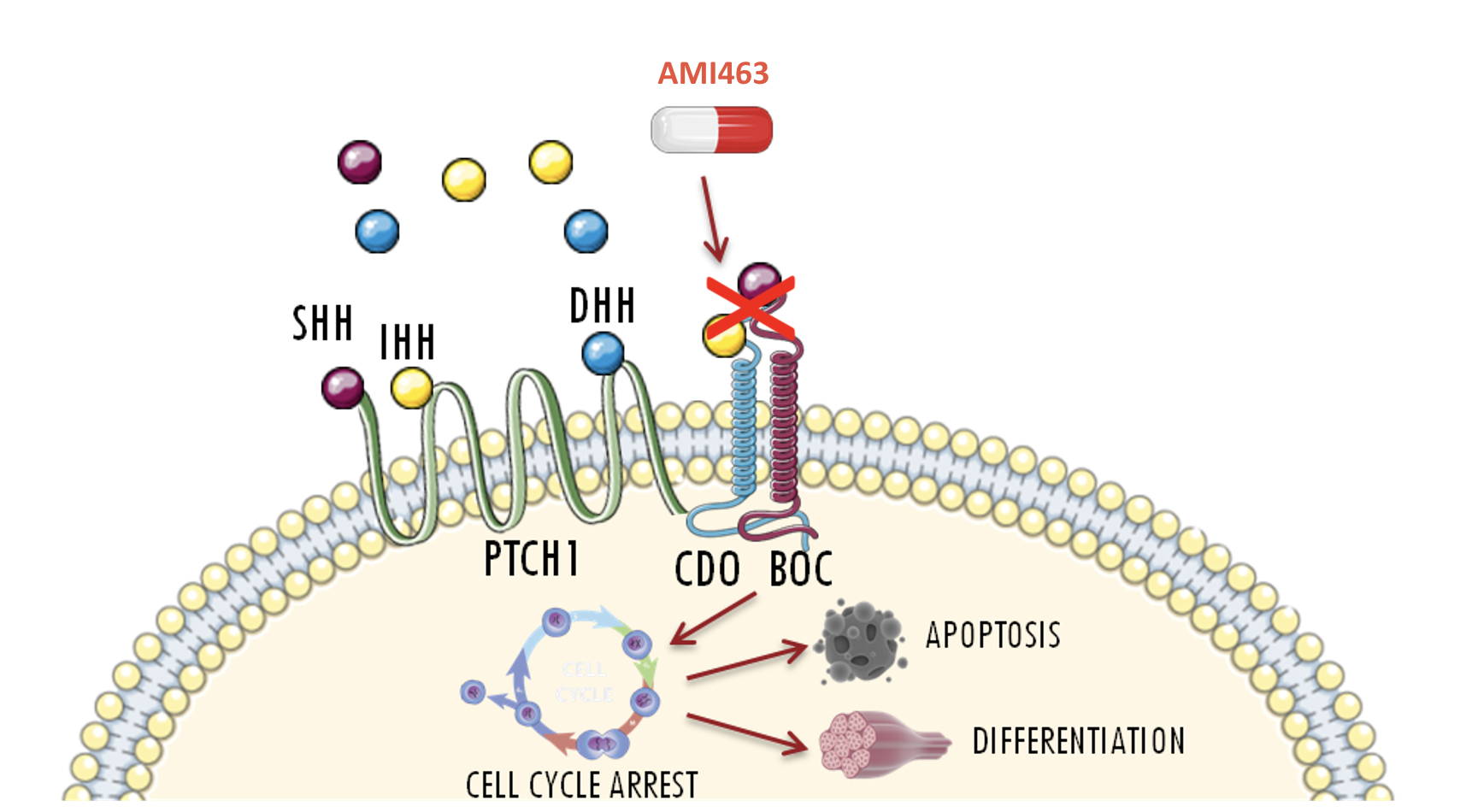
AMI463 is the first selective inhibitor of the Hedgehog (Hh) pathway coreceptor, CDO.
New Target
CDO is a new therapeutic target against the Hedgehog pathway in rhabdomyosarcoma and other pediatric cancers.
Specificity
CDO is a receptor with high tumor specificity in childhood cancers.
Potency
AMI463 has much greater antiproliferative potency than current Hh inhibitors.
Mechanism of Action
AMI463 arrests the cell cycle, induces apoptosis, promotes differentiation, and reduces the invasive abilities of cancer cells.
Efficacy
AMI463 shows antioncogenic efficacy in several cancer cell lines, standing out in the most aggressive subtype (PAX3/FOXO1 translocated).
Safety
AMI463 has no apparent toxicity not in cell lines nor in murine models.
Tumor reduction
AMI463 reduces orthotopic primary tumor growth by half in murine in vivo models.

AMI463 is an investigational selective CDON inhibitor.
Dysregulation of the Hedgehog pathway has been shown to occur in many cancer types and it is one of the most commonly mutated oncogenic pathways in cancer.
AMI463 is an investigational, brain-penetrant, highly-selective Cell adhesion molecule (CAM)-related downregulated by oncogenes (CDON) designed to target a key enzyme in the MAPK signaling pathway, which may offer an important alternative for people with primary brain tumors or brain metastases of solid tumors.
AMI463 has been meticulously tested in over ten different animal models, showcasing promising outcomes. Amira Therapeutics is currently preparing for a pivotal Phase 1/2 clinical trial of AMI463 to assess its safety and efficacy in treating Rhabdomyosarcoma (RMS), the most common form of soft tissue cancer in children.
Development Partnered with the Vall d'Hebron Research Institute (Barcelona)

Contact Us.
Email Us
info@amiratx.com
Call Us
+34937882300
Office
C/ Innovació, 2 (Leitat Technological Center).
08225 Terrassa (Barcelona), Spain.

